Role of neutrophil NETs in inflammatory diseases
Head: Prof. Dr. rer. nat. Dr. med. habil. Martin Herrmann
We are interested in understanding the role of neutrophil extracellular traps (NETs) in the pathogenesis of inflammatory diseases. NETs are decondensed chromatin filaments that neutrophil granulocytes eject from their nuclei after various stimulations. NETs can immobilize pathogens and thus prevent their spread throughout the organism. On the other hand, NETs are also involved in many pathologies. They can clog blood vessels, especially end-stream capillaries, and the ducts of exocrine glands. This can lead to chronic inflammation. Since NETs carry many enzymes and other proteins on their surfaces, they can influence many important reactions.
Source: Uni-Klinikum Erlangen
- NETs in bacterial infections and mucoses
NETs are involved in a variety of bacterial infectious diseases. They are induced, among others, by triggering TLR4 with LPS and various other TLR ligands and triggering FcgR with immune complexes. In this context, the formation of NETs is linked to the enzymatic synthesis of reactive oxygen (ROS) and the binding of granular enzymes to the massive decondensed chromatin. NETs are thus supercell-sized bioreactors. In healthy individuals, they have a short half-life, which reduces collateral damage. In various diseases, however, NETs remain active for a long time, which can lead, for example, to uncontrolled proteolysis and thus to damage of the extracellular matrix, which in turn causes functional impairment of entire organs. We also study NETs particularly in the granulomas of patients with tuberculosis and in the fluid from dental pockets of patients with periodontosis.
- NETs in blood vessels
In steady state, only very few neutrophils form intravascular NETs, which are easily cleaved by the circulating nucleases DNase-1 and DNase1L3 and then degraded in the liver. After strong activation of the immune system, as found for example in septic clinical pictures, massive uncontrolled NETs formation may occur. This overwhelms the circulating nucleases and can lead to clogging of the blood vessels. These immune thrombi are quite different from canonical thrombi. We are currently studying the metabolism of fibrin-NET complexes using material removed during surgery and in vitro generated immunothrombi.
- NETs in the ducts of exocrine glands
The ducts of exogenous glands are also monitored by neutrophils. They migrate into the berry-shaped secretory terminal of the glands, the so-called acinus, and are then passively transported in the secretion to the duct mouth. Because many secretions contain high concentrations of bicarbonate, neutrophils form NETs. They bind retrogradely invaded pathogens and transport them to the orifice with the flow of secretions. Under inflammatory conditions, migration of large amounts of neutrophils may occur, and their NETs aggregate and clog the ducts. In this field we work on salivary gland, lacrimal gland, meibomian gland, pancreas and the bile.
- NETs in patients with COVID-19
In sections from autopsy material, we observed a large number of occluded blood vessels of very different sizes. Both capillaries and larger vessels were affected. These atypical neutrophil-induced thrombi cannot be prevented with canonical anticoagulation as used in intensive care units. They respond inadequately to the lysis system. We are currently investigating the mechanisms by which these immune thrombi are triggered, which are causally related to severe disease progression.
- Ectopic calcification
DNA is an essential component of NETs. It has a sugar-phosphate backbone consisting of highly repetitive phosphate groups that can monovalently bind Ca++ to form a microscopic nucleus for the formation of hydroxyapatite. This nucleus can grow by further accumulation of Ca++ and PO43-- and eventually form visible crystal aggregates. We are currently investigating the factors that drive, or inhibit, ectopic biocalcification. We are analyzing soft tissue calcifications, such as calcinosis cutis, as well as the formation of stones in the gallbladder, pancreas and kidney.
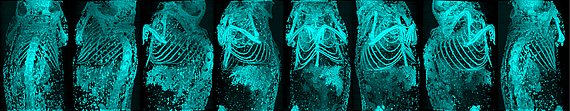
- Detection of clogged capillaries via endogenous fluorescence
In fluorescence microscopy, we observed that unstained capillaries in tissue biopsies, when excited at 488nm, emit a strong signal at 520nm when occluded. Thus, these pathologically important occlusions of the end-stream region can be easily detected and clearly distinguished from healthy, open capillaries.
- Agonistic antibodies against G-coupled receptors
In various diseases, autoantibodies occur that permanently and unregulated stimulate G-protein coupled receptors and thus strongly interfere with important regulatory circuits of the organism. For example, dilated cardiomyopathy is associated with antibodies against the β1 adrenergic receptor. It is considered certain that they can influence, or even trigger, the disease process. Currently, we are investigating agonistic autoantibodies in patients with COVID-19 and their involvement in the disease pattern "Long COVID".
| Martin Herrmann | Research group leader |
| Jasmin Knopf | Postdoctoral researcher |
| Sami Hosari | Postdoctoral researcher |
| Lei Liu | Postdoctoral researcher |
| Irmgard Herrmann | Image editing |
| Jeeshan Singh | Scientific doctoral student |
| Leticija Zlatar | Scientific doctoral student |
| Han Wang | Medical doctoral student |
| Maximilian Dölling | Medical doctoral student |
| Daniel Hosari | Medical doctoral student |
Deutsche Forschungsgemeinschaft (DFG)
FOR 2886 - PANDORA "Sodium-dependent tissue osmolarity in the control of rheumatoid arthritis." (2022-2025)
SFB/TRR 241 "Emergency barriers-neutrophil networks (NETs) and their interaction with intestinal epithelium." (2018-2022)
CRC 1181 "Amplification of NET formation and neutrophil aggregation for the resolution of inflammation." (2019-2023)
European Commission
ERC Grant "NeutroCure - Development of “smart” amplifiers of reactive oxygen species specific to aberrant polymorphonuclear neutrophils for treatment of inflammatory and autoimmune diseases, cancer and myeloablation." (2020-2024)
- Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C, Kremer AN, Völkl S, Amann K, Evert K, Falkeis C, Wehrfritz A, Rieker RJ, Hartmann A, Kremer AE, Neurath MF, Muñoz LE, Schett G, Herrmann M. (2020) Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 58:102925.
- Muñoz LE, Boeltz S, Bilyy R, Schauer C, Mahajan A, Widulin N, Grüneboom A, Herrmann I, Boada E, Rauh M, Krenn V, Biermann MHC, Podolska MJ, Hahn J, Knopf J, Maueröder C, Paryzhak S, Dumych T, Zhao Y, Neurath MF, Hoffmann MH, Fuchs TA, Leppkes M, Schett G, Herrmann M. (2019) Neutrophil extracellular traps initiate gallstone formation. Immunity. 51(3):443-450.e4.
- Boeltz S, Amini P, Anders HJ, Andrade F, Bilyy R, Chatfield S, Cichon I, Clancy DM, Desai J, Dumych T, Dwivedi N, Gordon RA, Hahn J, Hidalgo A, Hoffmann MH, Kaplan MJ, Knight JS, Kolaczkowska E, Kubes P, Leppkes M, Manfredi AA, Martin SJ, Maueröder C, Maugeri N, Mitroulis I, Munoz LE, Nakazawa D, Neeli I, Nizet V, Pieterse E, Radic MZ, Reinwald C, Ritis K, Rovere-Querini P, Santocki M, Schauer C, Schett G, Shlomchik MJ, Simon HU, Skendros P, Stojkov D, Vandenabeele P, Berghe TV, van der Vlag J, Vitkov L, von Köckritz-Blickwede M, Yousefi S, Zarbock A, Herrmann M. (2019) To NET or not to NET: current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 26(3):395-408.
- Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, Kluge S, Panzer U, Mizuta R, Mannherz HG, Kitamura D, Herrmann M, Napirei M, Fuchs TA. (2017) Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 358(6367):1202-1206.
- Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin NY, Dietel K, Bozec A, Herrmann M, Kaplan MH, Weigmann B, Zaiss MM, Fearon U, Veale DJ, Cañete JD, Distler O, Rivellese F, Pitzalis C, Neurath MF, McKenzie ANJ, Wirtz S, Schett G, Distler JHW, Ramming A. (2017) Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med. 23(8):938-944.
- Pfeifle R, Rothe T, Ipseiz N, Scherer HU, Culemann S, Harre U, Ackermann JA, Seefried M, Kleyer A, Uderhardt S, Haugg B, Hueber AJ, Daum P, Heidkamp GF, Ge C, Böhm S, Lux A, Schuh W, Magorivska I, Nandakumar KS, Lönnblom E, Becker C, Dudziak D, Wuhrer M, Rombouts Y, Koeleman CA, Toes R, Winkler TH, Holmdahl R, Herrmann M, Blüml S, Nimmerjahn F, Schett G, Krönke G. (2017) Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. 18(1):104-113
- Muñoz LE, Bilyy R, Biermann MH, Kienhöfer D, Maueröder C, Hahn J, Brauner JM, Weidner D, Chen J, Scharin-Mehlmann M, Janko C, Friedrich RP, Mielenz D, Dumych T, Lootsik MD, Schauer C, Schett G, Hoffmann M, Zhao Y, Herrmann M. (2016) Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proc Natl Acad Sci U S A. 113(40):E5856-E5865.
- Leppkes M, Maueröder C, Hirth S, Nowecki S, Günther C, Billmeier U, Paulus S, Biermann M, Munoz LE, Hoffmann M, Wildner D, Croxford AL, Waisman A, Mowen K, Jenne DE, Krenn V, Mayerle J, Lerch MM, Schett G, Wirtz S, Neurath MF, Herrmann M*, Becker C*. (2016) Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat Commun. 7:10973. *shared senior authorship
- Harre U, Lang SC, Pfeifle R, Rombouts Y, Frühbeißer S, Amara K, Bang H, Lux A, Koeleman CA, Baum W, Dietel K, Gröhn F, Malmström V, Klareskog L, Krönke G, Kocijan R, Nimmerjahn F, Toes RE, Herrmann M, Scherer HU, Schett G. (2015) Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 6:6651.
- Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, Lell M, Manger B, Rech J, Naschberger E, Holmdahl R, Krenn V, Harrer T, Jeremic I, Bilyy R, Schett G, Hoffmann M, Herrmann M. (2014) Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 20(5):511-7.
PubMed publication list of Prof. Dr. Martin Herrmann
